Yeast Contamination
Given yeast’s ability to grow at low pH levels (acidic), low water activity (requires little water to reproduce), and even in the presence of some chemical preservatives, it has become a common food and pharmaceutical contaminant which can cause huge losses for companies and illnesses in consumers. Testing for yeast contamination is easy with Trace Analytics.
Yeast Contamination – Air, Gas, and Surface Testing
Yeasts are unicellular microorganisms that are used in a variety of ways in manufacturing. They can be particularly beneficial to pharmaceutical and food manufacturing facilities. Yeast can ferment sugar for the production of ethanol and carbon dioxide, commonly used for the production of bread and beers. Popular in bakeries and beverage companies as a leavening agent or fermentation ingredient, yeast can also be found in the mouth, intestinal tract, and other parts of the human body. Pharmaceutical companies have bioengineered yeast strains to mass-produce insulin and other life-saving drugs.
Despite yeasts’ many productive uses, unwanted yeast contamination or an overgrowth of the microorganism can be detrimental to manufacturing processes and harmful to products. Regular monitoring and microbial testing are critical to ensuring that yeasts are not negatively impacting products.
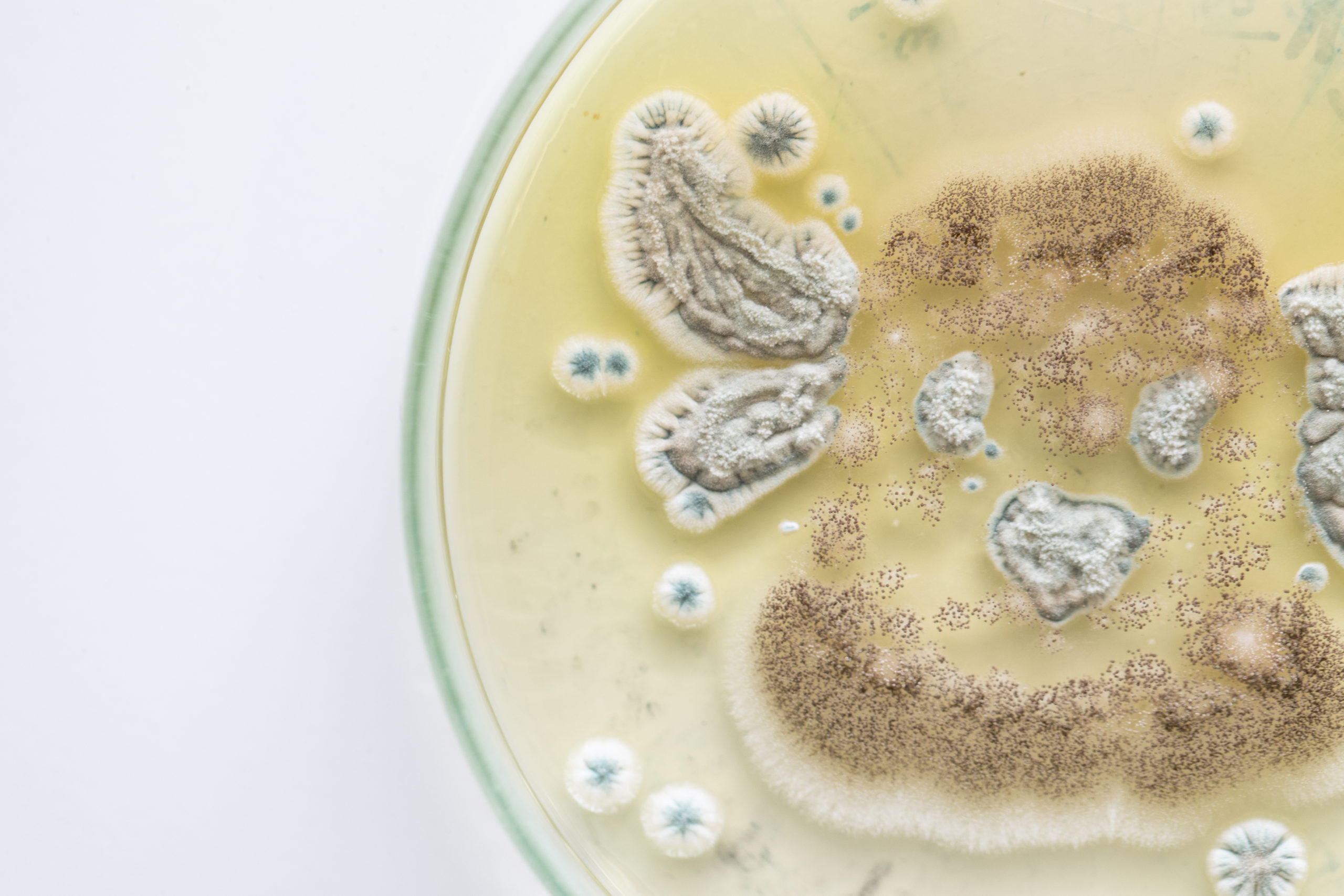
Who Is At Risk of Yeast Contamination?
Food and beverage manufacturers need to be particularly cautious of yeast contamination as this microorganism grows particularly well in sugar, and though growth is slowed, it can grow in the presence of salt as well. Yeasts range from a few micrometers (typically) up to 40 micrometers (rarely).
Fungal contamination, especially yeast, in pharmaceutical products represents two hazards. First, it can cause product spoilage. Yeast can convert the sugars in medicines into by-products, which alter the product. If the profiles of a medication change, then the products cannot be sold. Secondly, product contamination represents a health hazard to the patient, potentially causing disease, depending on the route of administration and the patients’ resistance to infection.
Consequences of Yeast Contamination
Both yeasts and molds cause various degrees of deterioration and decomposition of foods. They can invade and grow on virtually any type of food at any time. Some important food spoilage yeast species are Zygosaccharomyces, Candida, Saccharomyces, Debaryomyces, and Brettanomyces. If yeasts are identified improperly and treated as bacterial contamination, then they can become resistant to decontamination processes.
In March 2018, the FDA reported a voluntary recall of four lots of skin protectant topical cream due to confirmed microbial contamination with high levels of yeast, mold, & bacteria. The microbes altered the product’s properties resulting in huge losses both financially and in brand reputation.

Given yeast’s ability to grow at low pH levels (acidic), low water activity (requires little water to reproduce), and even in the presence of some chemical preservatives, it has become a common food and pharma contaminant which can cause huge losses for companies and illnesses in consumers.
Proper monitoring programs and remediation plans are critical in controlling yeast growth. Because yeasts grow so abundantly, are used so commonly, and thrive in so many different environments, it’s important to monitor and test regularly to ensure the safety of all end products
Conditions for Yeast Contamination
Both environmental and compressed air points-of-use must be regularly monitored for bacteria, yeast, and mold. Little water is required to support the growth of yeast and so it is critical to dry the compressed air or gas for end-use. If lubricated air is required, then condensation and drip pans around the compressor itself should be dry and cleaned routinely.
Yeasts thrive in air handling units and wet pipes. They are often brought into a facility on employee clothing or under shoes. Facilities can reduce the number of microorganisms by controlling the environmental conditions in the working facility and near the air compressor or compressed gas inlets and outlets.

Testing Made SimpleAccurateEasy
Trace Analytics’ laboratory is accredited by the American Association for Laboratory Accreditation. We use state-of-the-art lab equipment that allows us to analyze hundreds of compressed air and environmental samples daily. The result is consistency, accuracy, precision, and rapid turnaround. Trace is an A2LA accredited laboratory complying with ISO 17025, certificate #0322-01.