Original article published on PharmaManufacturing.com
Pharmaceutical manufacturers can apply cleanroom standards to compressed air systems to ensure the quality and safety of their end-products. Often overlooked due to lack of specific regulations, compressed air systems are critical to many processes in a cleanroom environment. Cleanroom specifications can be applied to compressed air systems and included in monitoring plans for year-round quality assurance.
Recommendations on Compressed Air Quality
The International Society for Pharmaceutical Engineers (ISPE) Good Practice Guide, recommends, “in cases where the gas is entering a classified area, it is required to at least meet the room classification limits established for the cleanroom environment” (2016). Additionally, the most recent US FDA Guidance for Industry Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice recommends that “compressed gas should be of appropriate purity… and it’s microbiological and particle quality after filtration should be equal to or better than that of the air in the environment into which the gas is introduced.”
Using the US FDA and ISPE GPG recommendations, pharmaceutical manufacturers can properly evaluate the quality of their processed gases including nitrogen, oxygen, argon, carbon dioxide, and compressed air. Chad Larrabee, Global Product Management Leader of Ingersoll Rand and GPG: Process Gases co-chair, explains that the compressed air used in cleanroom environments should match the quality levels required by that room (2019) as mentioned by the FDA.
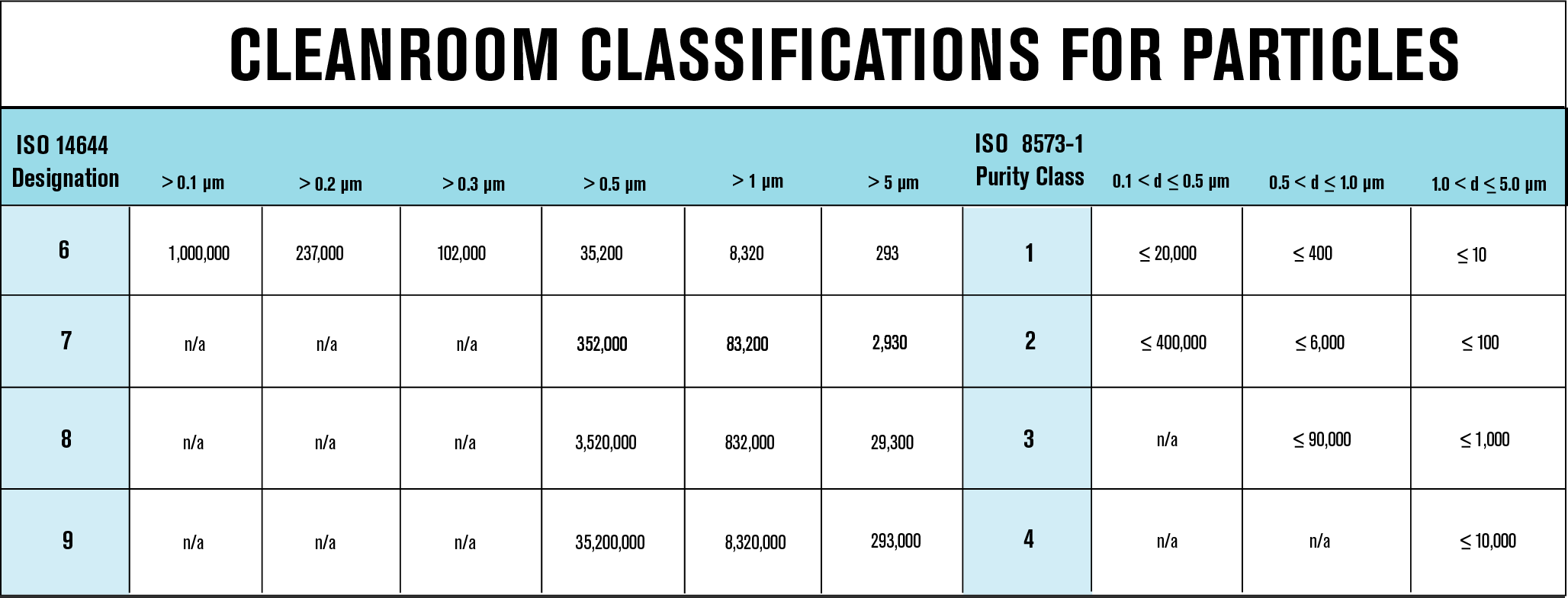
The International Organization for Standardization (ISO) standard, ISO 8573:2010 is commonly used for compressed air applications across a variety of industries. Pharmaceutical manufacturers often favor cleanroom specifications over ISO 8573-1 to adhere to their cleanroom facility. To facilitate communication between manufacturers and distributors, ISO 8573-1 specifications can be translated into cleanroom specifications. The following chart demonstrates the comparison between both standards and how they can be used together to prevent confusion.
Common Uses of Compressed Air and Process Gases
Process gases and compressed air are used in a variety of ways depending on the product manufactured. Compressed air can be used in direct contact with products to clean, aerate, or move them through the processes. It can also be used for packaging. Process gases can be used in fluid pumps that take products through the production and filling processes. Process gases can also spray or coat a product, or act as an ingredient of the product itself.
To identify risks for creating a monitoring plan, consider how compressed air or gas is used, along with the amount and type of contact they have with products.
Contamination and Risks
Common contaminants in process gas or compressed air:
- Particles (non-viable)
- Water
- Oil
- Microorganisms (bacteria, yeast, mold)
These can put products and systems at risk and require regular testing.
Particle quantities in ambient or environmental air can be measured using cleanroom specifications. Compressed air becomes contaminated by drawing in unfiltered ambient air where particles, water, oil, and/or microorganisms enter the system through the intake.
In order to prevent shutdowns or loss of revenue and consumer trust, pharmaceutical manufacturers can implement proper filtration, desiccant dryers, and regularly test their compressed air with an accredited laboratory. These procedures can help manufacturers protect their products and company reputation.
Controlling and Monitoring Processed Gases and Compressed Air
According to Chad Larrabee, Global Product Management Leader of Ingersoll Rand, predictability and repeatability are the most important factors of any quality plan in pharmaceutical manufacturing. For this reason, it is necessary to design a monitoring and sampling plan that allows facilities to catch potential issues before damaging any products (Larrabee, 2019).
A risk assessment created by the facility can help determine sampling frequency and locations. It is important that manufacturers consider risks unique to their facility. Manufacturers can choose to test quarterly to account for seasonal changes, before and after maintenance is performed, or annually. Testing once per year satisfies current standard recommendations, but does not provide sufficient data needed to conduct a trend analysis.
Ingersoll Rand provides oil-free compressed air systems certified by TUV for class 0 according to ISO 8573-1:2010, meaning the compressor does not add oil to the air stream. However, because oil can still enter the compressor’s intake through the atmosphere. Regular testing with an accredited laboratory can help manufacturers ensure that no contaminants have entered the compressed air system.
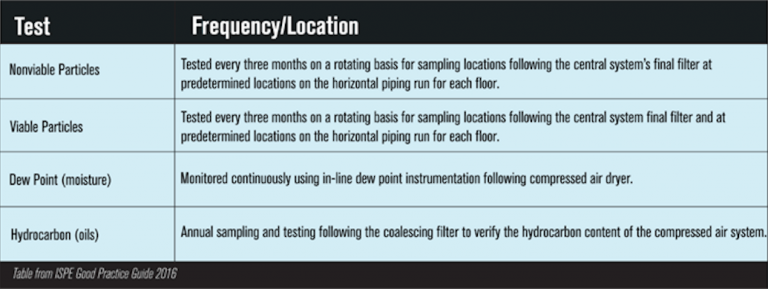
The ISPE Good Practice Guide provides the following chart as a helpful recommendation of sampling using cleanroom specifications for compressed air plans per contaminant:
Meeting Cleanroom Specifications for Compressed Air Systems
Working with an accredited laboratory can ensure that your facility’s individual sampling and testing needs are met. It is critical that manufacturers meet cleanroom specifications through specific purchase or rental equipment and that analysis reports are received in a timely manner. Trace Analytics, LLC can test to a wide variety of specifications, including ISO 8573-1, ISPE Good Practice Guide, Cleanroom Classifications and custom specifications, in addition to providing quick, reliable analyses.