Compressed air is a critical utility that is widely used throughout the food industry. Many of the GFSI schemes like Safe Quality Food (SQF), British Retail Consortium (BRC), primusGFS, and Food Safety System Certification (FSSC) 22000, require compressed air quality monitoring. FDA’s Food Safety Modernization Act (FSMA) requires covered manufacturing facilities to establish and implement a food safety system that includes an analysis of hazards and risk-based preventive controls.
Knowing which contaminants may be in compressed air is key to avoiding product contamination. Food manufacturers must assess the activities and operations that can harm a product, the extent to which a product can be harmed, and how likely it is that product harm will occur.
Assessing product contamination is a multi-step process in which you must identify the important risks, prioritize them for management, and take reasonable steps to remove or reduce the chance of harm to the product, and, in particular, serious harm to the consumer.
Identify Potential Hazards
Both the Compressed Air and Gas Institute (CAGI) and ISO 8573 specify compressed air contaminants. CAGI cites ten contaminants that typically need to be removed or reduced from low-pressure compressed air used for manufacturing. These ten contaminants fall into four general categories.
Four General Categories of Contaminants:
- Particles (pipe scale, wear particles from the compressor operation and atmospheric dirt)
- Water (liquid, vapor and aerosol form)
- Oil (liquid, vapor and aerosol form)
- Microorganisms and non-viable particles
IFSQN Webinar, Compressed Air System Risk Assessment: Do I Need to Test?
Sources of Contamination that Can Cause Product Harm:
- Compressor type (oil)
- Dryer type
- Distribution piping
- Storage receivers
- Filters
- Leaks
Compressor Points-of-Use that Need to be Monitored:
- Valves
- Gauges
- Flexible tubing
- Fittings
- Filter maintenance
Air Quality Standards
Currently, there are very few published air quality standards for manufacturers. Regulating and certifying bodies like SQF, FSMA, and FDA require manufacturers to monitor compressed air, establish purity limits, and ensure that the air does not add contamination to the final product. But they do not define the exact specification for the quality of the compressed air.
ISO 8573-1:2010 is the international standard used as a common language for compressor and filter manufacturers, food manufacturers, and laboratories. It is an effective standard that can be applied across many countries and facilities to define and establish contaminant purity specifications for compressed air.
ISO 8573-1 states, “The three major contaminants in compressed air are solid particles, water and oil; these are categorized by compressed air purity classes. These compressed air purity classes group the concentrations of each of the above contaminants into ranges, each range being given its own purity class index. The range limits are aligned to those figures found in practice.”
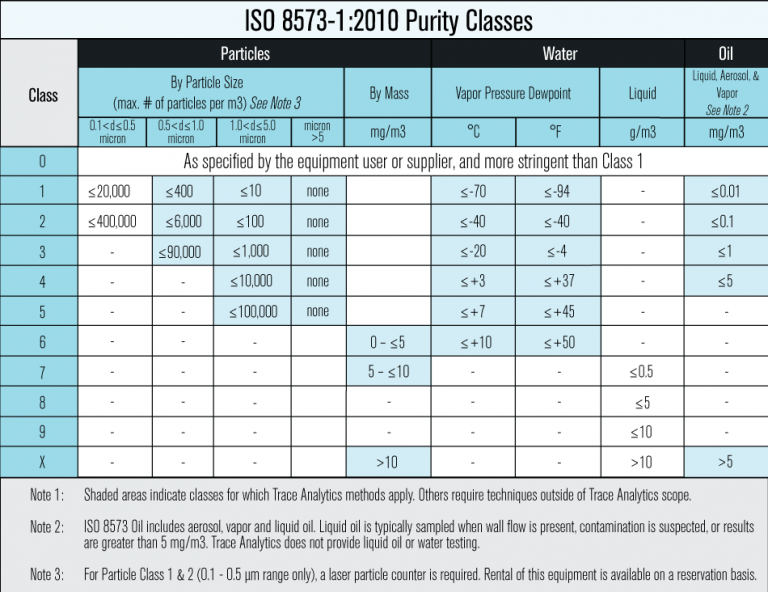
Designating ISO 8573-1:2010 Purity Classes
The designation of ISO 8573 purity classes include the specification name and edition date, the purity class number in brackets, and is always listed in the order of Particles, Water, and Oil with the numbers being separated by a colon: ISO 8573-1:2010 [Particles:Water:Oil]
ISO 8573 provides a sufficient range of purity limits and gives manufacturers some flexibility when determining a product’s purity class. The British Compressed Air Society (BCAS), with input from the British Retail Consortium (BRC), produced the guide below providing purity class recommendations for the food and beverage industry.
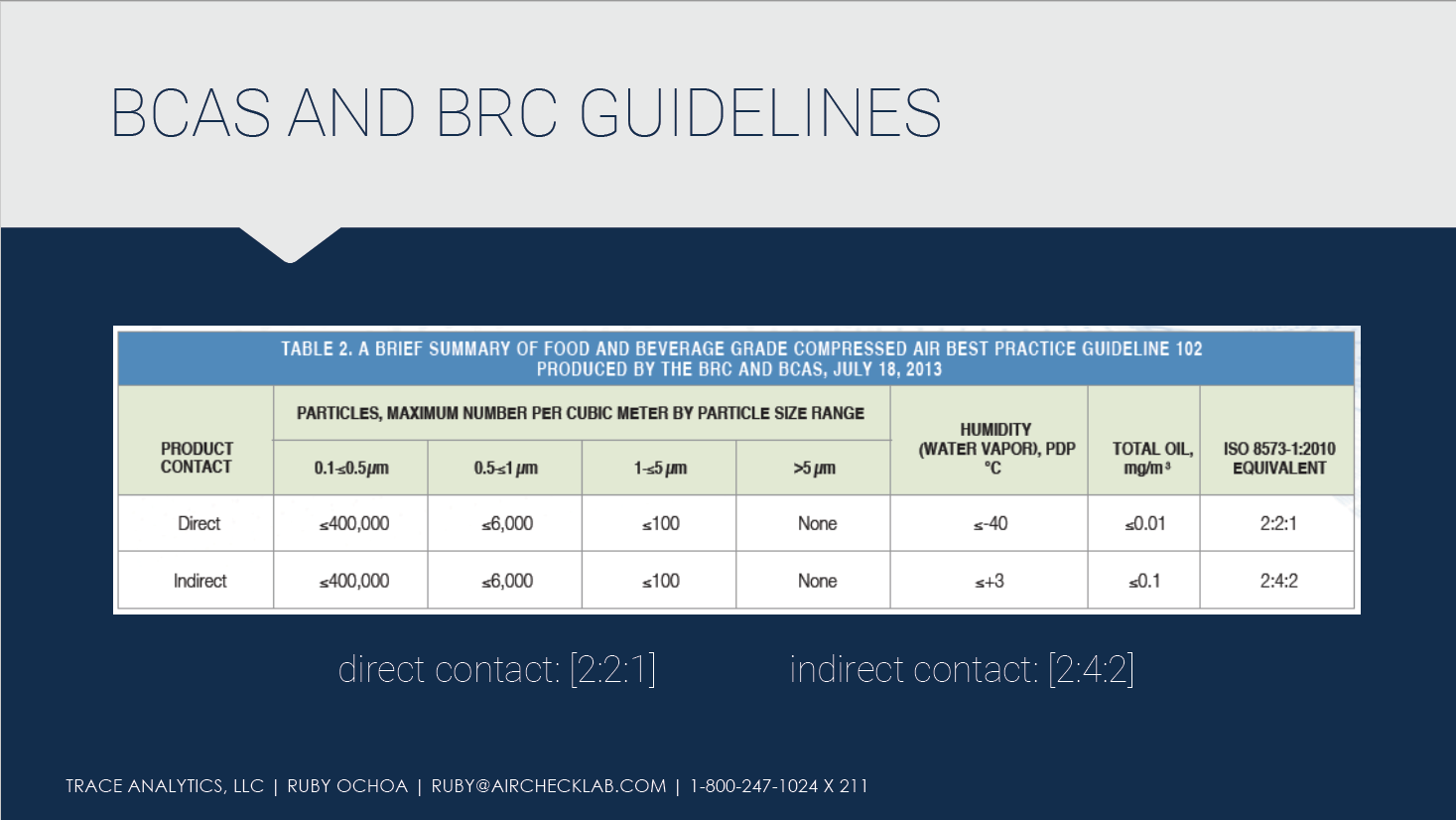
According to the BCAS Guideline, compressed air quality shall be tested and verified at least twice per year or per the manufacturer’s recommendations. Additional testing is also warranted whenever maintenance work or any activity that may affect the air quality is performed on the compressed air system.
Conduct a Risk Assessment
A risk assessment checklist can be used to assess the health of a compressed air system.
The checklist helps manufacturers document information about the compressor, the installed filtration, the points-of-use and the sampling port connections. Once this information is collected manufacturers are able to assign an appropriate level of risk with a numerical value (with 0 being no risk and 5 being certain risk) to each element of the compressor.
For your copy of the blank risk assessment click here.
A risk assessment is a crucial step recommended by both SQF and HACCP. See how to apply HACCP to your compressed air system here.
Assess Risk of Harm
Once the risk assessment is complete, manufacturers need to assign levels of risk to the compressed air system.
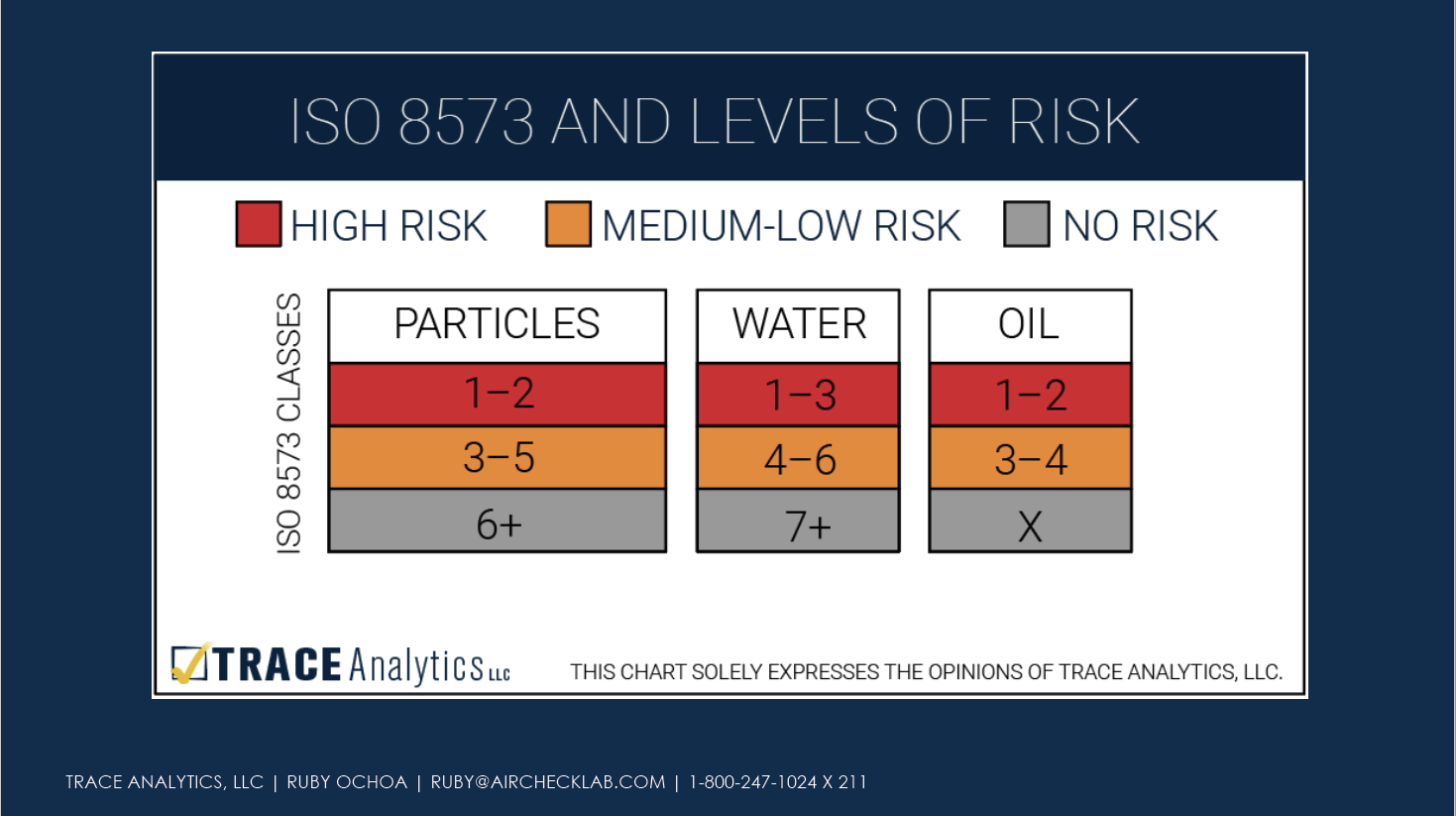
Set Purity Limits
After assessing risk across the entire compressed air system, manufacturers will synthesize this information with the needs of their particular products and establish purity limits. Without setting purity limits based on risk of product harm, manufacturers cannot adequately assess existing controls, assess if extra controls are needed, or determine an appropriate monitoring plan.
Setting purity class limits can be very difficult. It requires manufacturers to take the information about the compressed air system gathered during the risk assessment and apply that information directly to their product. Manufacturers are not required to have the same purity limits for all their products. The limits can vary from product line to product line if so determined by the risk assessment.
Click here for more information about how to designate purity classes.

Assess Existing Controls & Review Efficacy of Controls
Once a risk assessment is completed and the product’s purity limits are established, current controls must be monitored to guarantee that they are performing to the standards of the purity limits set.
Where Do You Test?
Results from air samples should be representative of the air used on your products. Capture samples as close to the point-of-use filtration and identify any critical control points. Take sufficient samples that cover all of the variations in your air system.
Create a Monitoring Plan
There are several sampling options to consider when assessing your system and its controls.
• Percentage: Pick a percentage of the compressors outlets (25%, 33%, 50% etc.) to sample annually. We recommend sampling throughout the year to include seasonal changes, production variations, and maintenance schedules.
• Maintenance: Take samples before and immediately after compressor filter changes and point-of-use filter changes. Also, sample after any major changes to the compressor or distribution piping. Data obtained after 3-4 filter changes can be used to establish a trend analysis determining a) that air quality is in a state of control, b) filters are being changed at appropriate intervals for your production levels, and c) major changes did not negatively affect the air quality. This strategy can also be combined with the Percentage Based sampling plan.
• Minimal: Take three or four samples; one as close to compressor filtration as possible, one at the furthest point from compressor filtration, and then one or two samples from somewhere in between the other two. You can review the results to see if there is any degradation of air quality the further the air travel away from the main filtration. This will also take into consideration possible piping contamination.
• Single: Take one sample from a critical control point. Select a different point-of-use each time you sample. Taking one sample is a good first step and will satisfy current minimum requirements for GFSI-required certification audits. While some may call this a monitoring plan it is not because it does not provide enough data to establish a trend analysis.
For more information on monitoring plans click here.
How Often to Test
• Annually: While taking samples once a year may be convenient, taking samples at various times of the year provides more data to better gauge and guarantee consistent air quality.
• Semi-Annually: BCAS’s Food & Beverage Best Practice Guideline 102 recommends semi-annual testing and also any time major work is performed on the compressed air system.
• Quarterly: A trend analysis can provide useful information on the state of compressed air quality at various times throughout the year. By noting filter changes, compressor maintenance (both routine and emergency), and sampling dates, manufacturers can track the quality of your compressed air by outlet or product line. This frequency can then be reduced once you have sufficient data supporting consistent air quality.
Assess If Additional Controls are Needed
Reviewing the results of your air tests will provide the information you need to assess if your controls are adequate. If samples fail to meet your established limits, you will need to either reassess if the limits were set inappropriately, or add additional controls, such as point-of-use filters.
Schedule Regular Review
Don’t Wait Until Right Before an Audit
Compressed air systems are not static, but dynamic—always changing. Component parts breakdown and malfunction, requiring maintenance or replacement, and there is not always an obvious indication that a device which is plugged in and running is not performing to standard. Regular testing hedges against the possibility of underperformance or non-performance.
Compressed air quality is a critical aspect of sanitation in the food industry. While regulation is still in its infant stages in some places, the core desire to protect consumers is enough to warrant regular air testing, as well as to ensure that equipment and processing environments are operating efficiently. Testing, while it does cost, stands to safeguard against the possibility of greater cost of damage or incident.
To watch the recorded International Food and Safety Webinar (IFSQN), Compressed Air System Risk Assessment: Do I Need to Test? click here.
For a copy of the IFSQN Webinar presentation slides click here.