Ambient Air Testing Options: Active or passive sampling
Many food, pharma, medical device, and packaging manufacturers are facing new requirements and regulations requiring the monitoring and testing of their ambient air quality in high-risk locations. Bodies like SQF, FDA, and ISO are often vague about what constitutes monitoring and what is required, pointing to the user to determine limits and frequency based on their individual risks. One of the most common questions we receive regarding ambient air testing is: should my facility choose to perform active or passive sampling? What are the benefits of each option? Though both passive and active sampling reveal bacteria, yeast, and mold contamination, they have pros and cons which must be carefully considered when constructing a monitoring plan for your client or facility. The ultimate goal of ambient air monitoring is to ensure that the air inside the facility is not a cause of contamination on the end-product. Air sampling, be it active or passive, gives invaluable information on the general environment in which your preparation is being manipulated (Cockcroft 2011).
Trace Analytics offers two forms of ambient air testing: active and passive. Active sampling is performed with an impaction sampler, the Trio.bas mono. This sampler draws in a specified amount of ambient air for the test. Passive sampling can be done in a number of ways, but at Trace Analytics, we perform this testing with settle plates. These plates are simply placed in a work location and left to rest for up to 4 hours. They collect any microorganisms that may fall on them during that time.
Passive Sampling: Settle Plates
Passive sampling with settle plates is a traditional method for sampling for microorganisms. Simply put, these plates are set out in a work area deemed high-risk through a thorough risk assessment. The plates should be out no longer than 4 hours as this can cause potential dehydration of the sampling media or create a film encapsulating the agar and preventing proper microbial growth.
Once the sampling is complete, the plates are wrapped in parafilm, labeled, and shipped back to the lab for incubation and analysis. Incubation times often range from 5-10 days depending on the specification you are trying to meet.
Passive sampling is inexpensive and simple to do because it doesn’t require any equipment. You can take tests in multiple locations all at the same time while work continues.
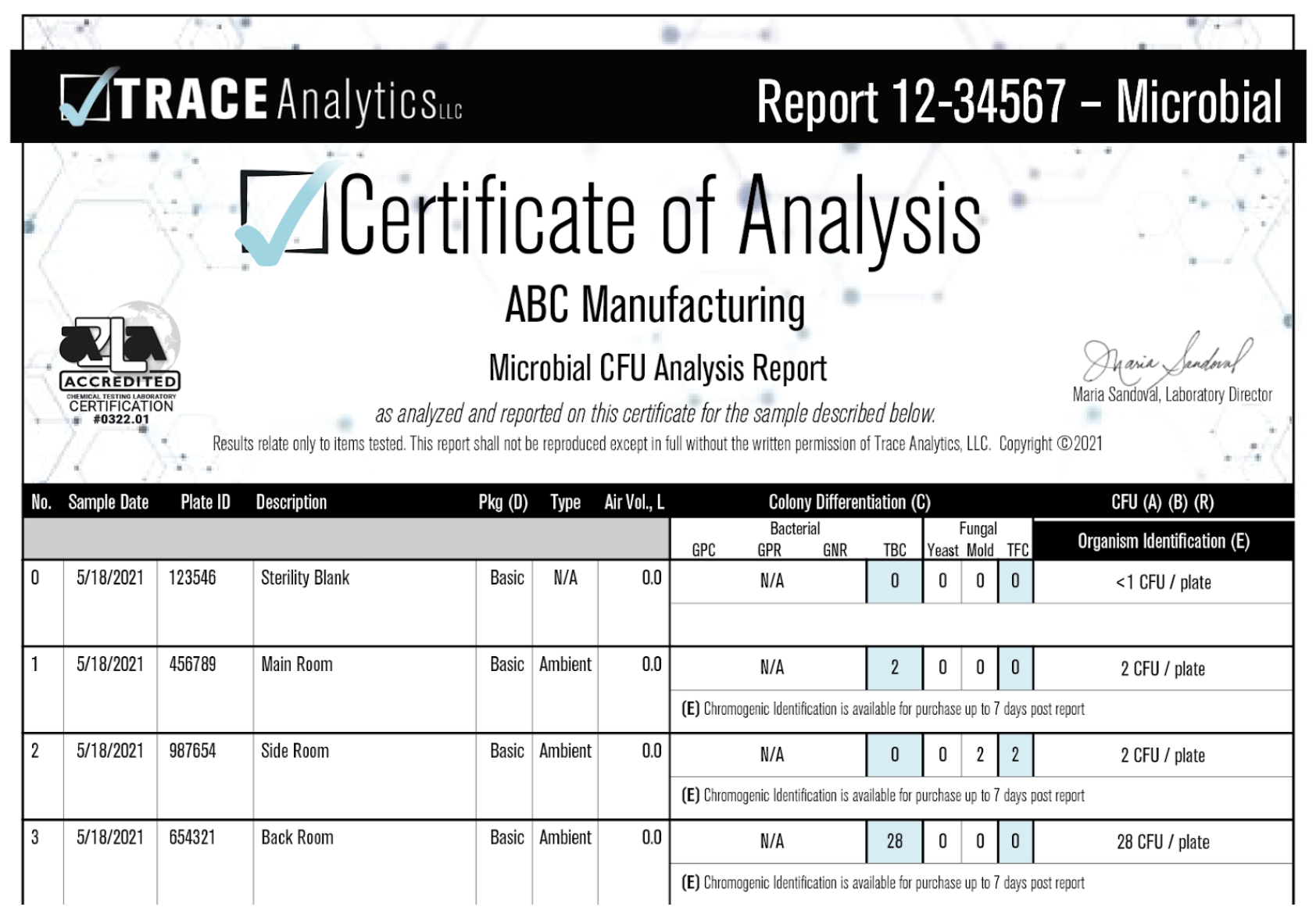
These results, however, are not quantitative as it is impossible to quantify the amount of air sampled with settle plates over that period of time. Your results will appear as CFUs (colony forming units) per plate on your reports.
Active Sampling: Impaction Sampler
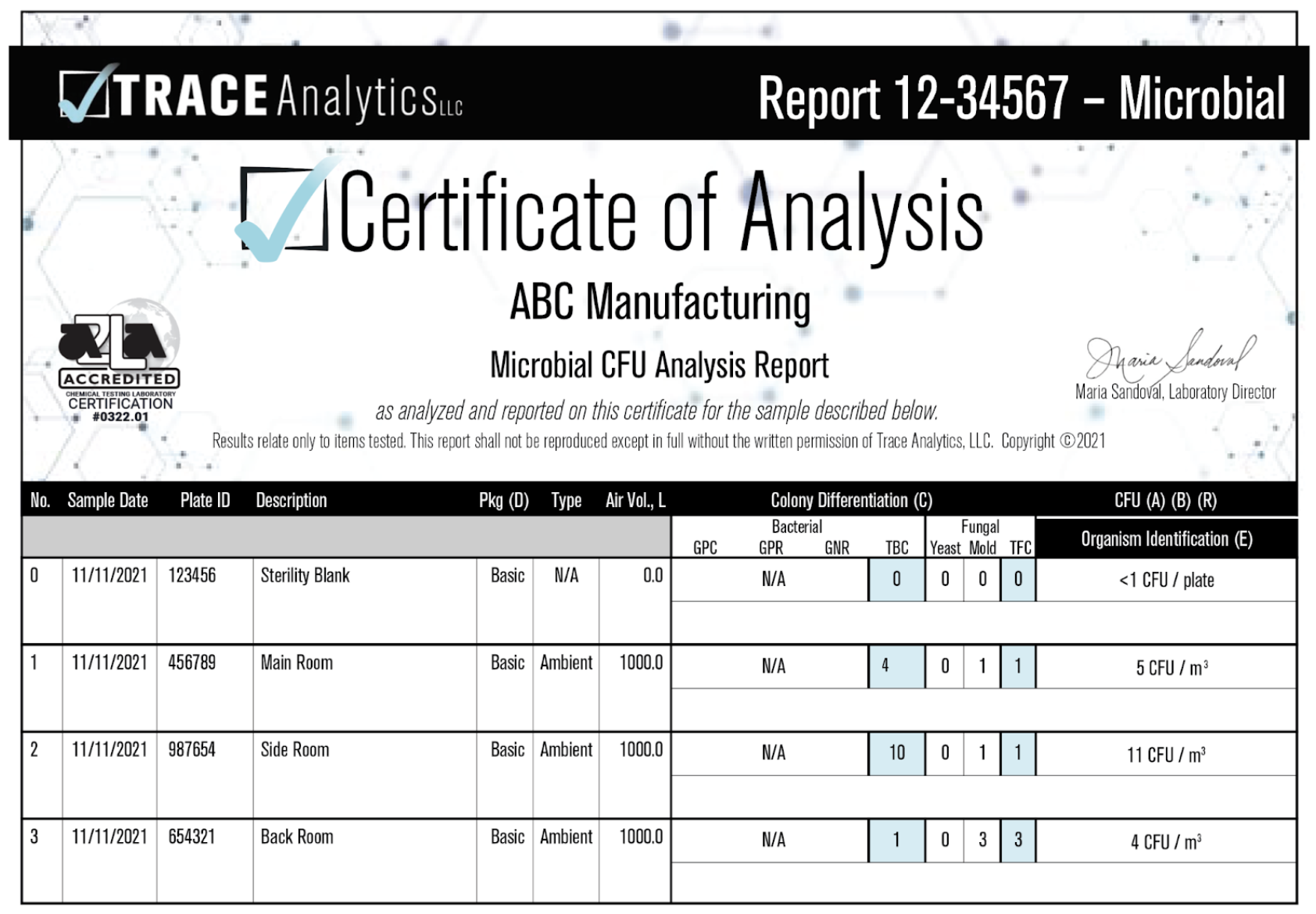
Active sampling with an impaction sampler is an excellent way to extrapolate statistical data and receive quantifiable results. The impaction sampler draws in an exact amount of air onto the agar plate. The analyst can then determine results per cubic meter or foot depending on their requirements. Results with an active impaction sampler are qualitative and quantitative. They will be reported as CFUs per cubic meter.
Active sampling is much faster than settle plate testing – usually the samples take about 5 minutes. While the equipment is easy to use, Trace Analytics offers detailed instructions and step-by-step videos to help guide you through the sampling process.
When active sampling is performed, the user must purchase or rent the equipment. If purchased, the owner must maintain calibration documentation for the instrument. Trace Analytics helps facilitate these requirements. Our lab has a fleet of Trio.bas mono samplers that we maintain and calibrate annually.
Results
Example Triobas Report
Example Settle Plate Report
Next Steps: Microbial Growth is Detected
If growth is detected on your plates during the incubation period, a member of our AirCheck team will reach out to you directly to determine if you’d like further identification. We can test any colony detected on the plates for genus and species.
Our 17025 accredited laboratory is able to identify microorganisms within its library of over 2,900 species using the Biolog. This system was created specifically for applications in quality control and environmental monitoring – focusing on microorganisms that are more prevalent and pertinent outside of the clinical setting. Facilities that choose to proceed with identification may choose to do so to meet their specification requirements, limit the possibility of product contamination, or confirm the efficacy of their aseptic technique.
Setting Up a Monitoring Plan
Regardless of the type of ambient air sampling that is chosen, a robust monitoring plan must be laid out to ensure the area in question is maintaining appropriate levels of quality to meet specifications. Crocroft states that it is recommended to monitor both when an environment is ‘at rest’ — i.e. equipment inoperative and unstaffed — and also ‘in use’, i.e. with routine activity in progress (2011). Because of this, it may be helpful to employ both active and passive sampling for some users, depending on the application.
Frequency of Ambient Air Testing
One of the most common questions that we are asked is “what should the frequency of my tests be?” The answer is entirely dependent on your particular facility, its needs, the end-product, and the specifications and requirements that you must meet.
Some clients sample monthly, some quarterly, others annually and even some daily. Baselining is an excellent way to help you make this decision. If an area is consistently low risk, you may consider reducing sampling frequency and vice versa until elevated results subside. We often recommend testing quarterly in high-risk areas. Testing at this frequency helps build trend analyses over time and will give you or your client adequate data to determine the source of contamination should it occur in the future.
Number of Microbial Samples
Another common question we receive is “how many samples should I take?”. Quantitative sampling can become very helpful. Because active samplers measure the amount of air that’s drawn into the sampler, you can determine home many samples need to be taken based on that amount. You can extrapolate the data for the size of your space if you know how much air has been sampled.
Again, this is a decision that should be made based on your risk assessment and specification requirements. You will want to make sure that you are appropriately testing the high-risk areas in your facility.
Microbial Limits
Just as with how frequently samples must be taken, when determining purity limits, some facilities prefer to take a “baseline” approach. Samples are taken periodically to look for major changes which may reflect a problem or change in their system that needs to be addressed. Other facilities choose purity limits based on their risk assessment to avoid the potential for contamination. On the other hand, ISO 14644 specifically states limits for particulates in the ambient air.
Monitoring and testing is intended to limit the potential for contamination of end-products. While some specifications have explicit purity and frequency requirements, not all of them do. If your chosen specification doesn’t include specific requirements it will be up to you to determine testing frequency and purity limits based on the potential for contamination in high-risk parts of your facility.
Trace Analytics has many resources available to help you or your client through the sampling and testing process. In addition to our risk assessment and monitoring plan documents, we also have a library of videos, webinars, and instructional documents readily available. At any point of the sampling process, our AirCheck Team of Experts is available to assist over the phone.
References:
Cockcroft, M. (2011, October 11). Environmental monitoring. Contract Pharma. Retrieved December 15, 2021, from https://www.contractpharma.com/issues/2011-10/view_features/environmental-monitoring/










